FDA Clears Onera Health’s At-home Sleep Diagnostic. Stated To Hit The US Market in 2023
Archive of the American Sleep Apnea Association
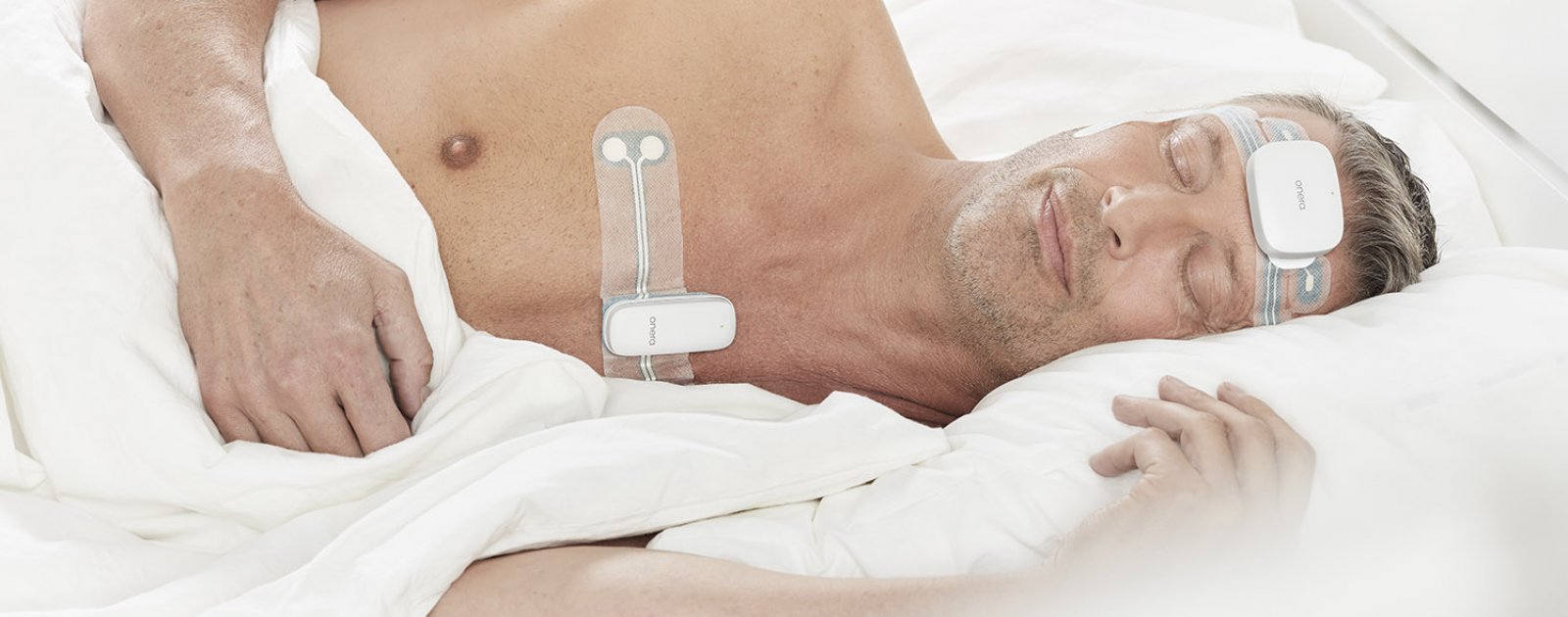
Based in the Netherlands, Onera’s patch-based technology promises to modernize the outmoded, time-consuming, and costly procedure of traditional sleep testing.
In a news release, Onera stated that their clinical-grade technology can provide an update to the traditional sleep testing procedure, which it claims is “partly responsible” for the underdiagnosis of sleep disorders.
The FD decided that the Onera STS system was “essentially equal” to Natus Medical’s commonly utilized device for in-lab PSG research.
The newly cleared method works in conjunction with Onera’s digital health platform to provide an end-to-end PSG service that permits and streamlines clinical-grade sleep studies with no upfront investment
.
Onera intends to commercialize the end-to-end system in Europe in the second part of this year.
Before full commercialization begins in 2023, the company aims to extend the launch with selected partnerships to the American market.